
Use the concept of effective nuclear charge to explain why the atomic radii of the main group elements increase when we move down a group in the periodic tableĪtoms Have No Definite Boundary.
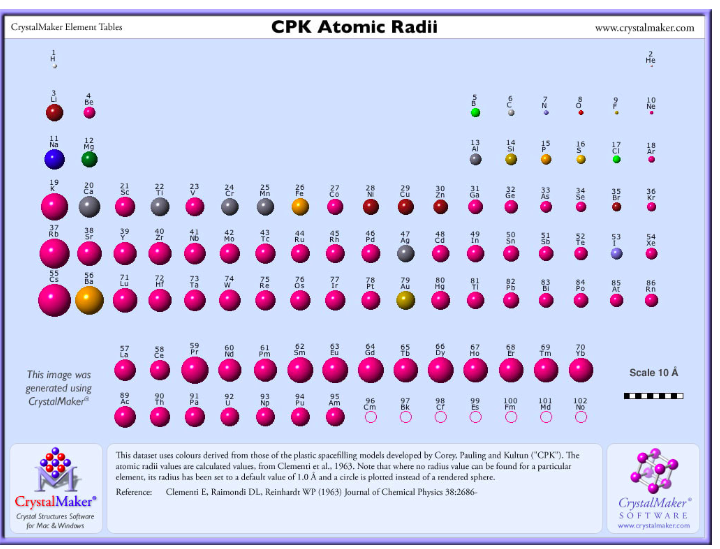
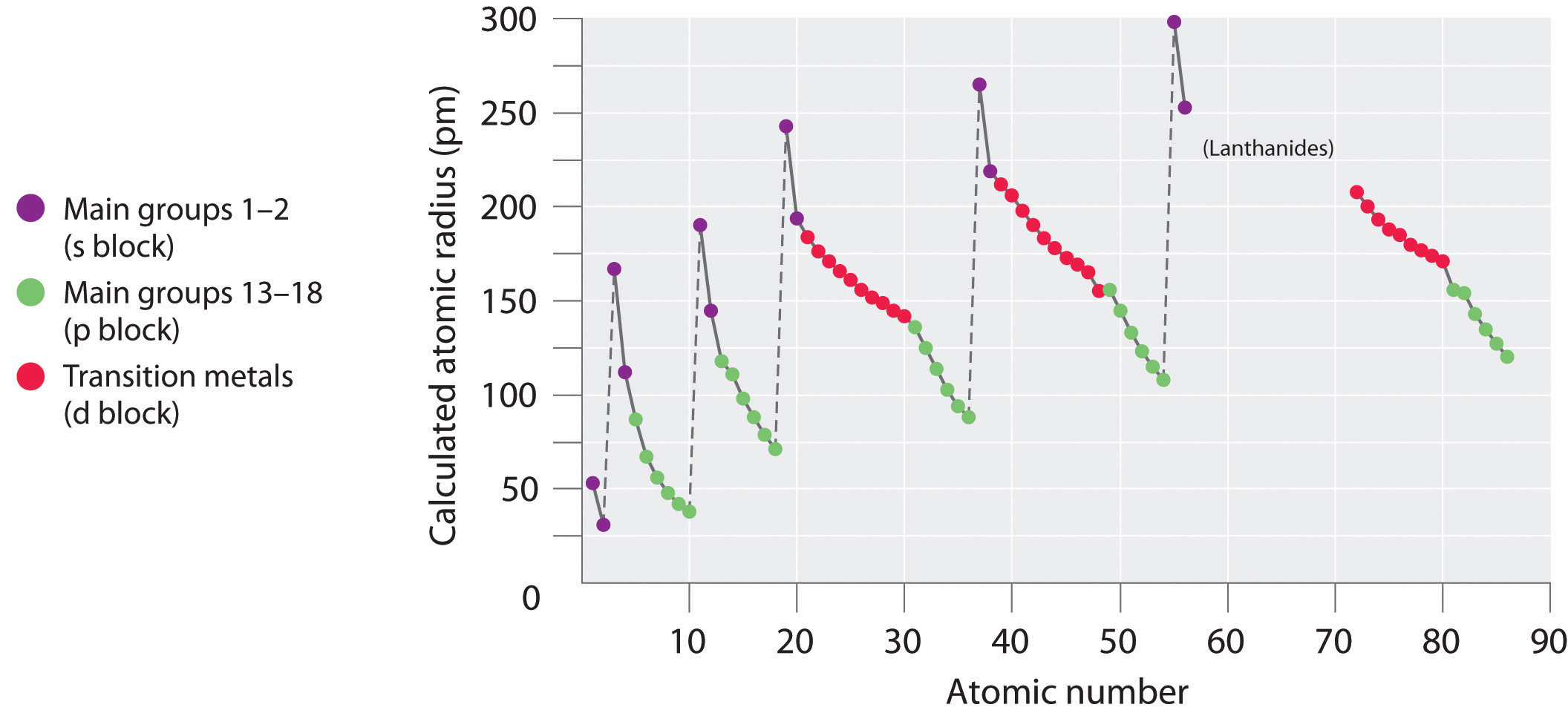
The actual trends that are observed with atomic size have to do with three factors. Each of these trends can be understood in terms of the electron configuration of the atoms. The two following this lesson will discuss ionization energy and electron affinity. The first lesson of this chapter is devoted to the trend in atomic size in the Periodic Table. There are three specific periodic trends that we will discuss. This means is that as you move down a group or across a period, you will see a trend-like variation in the properties. In the Periodic Table, there are a number of physical properties that are not really "similar" as it was previously defined, but are more trend-like. 5 For the Transition Elements, the Trend is Less Systematic.4 Atomic Size in a Period Decreases from Left to Right.3 Atomic Size in a Column Increases from Top to Bottom.


 0 kommentar(er)
0 kommentar(er)
